key point: Mass spectrometry is a technique for determining the mass of a molecule and of it fragments.
Mass spectrometry measures the mass-to-charge ratio of gaseous ions. The ions can be either positively or negatively charged, and it is normally trivial to infer the actual charge on an ion and hence the mass of a species. It is a destructive analytical technique because the sample cannot be recovered for further analysis.
Figure 1. A magnetic sector mass spectrometer. The molecular fragments are deflected according to their mass-to-charge ratio, allowing for separation at the detector.
The precision of measurement of the mass of the ions varies according to the use being made of the spectrometer (Fig. 1). If all that is required is a crude measure of the mass, for instance to within ±mu (where mu is the atomic mass constant, 1.66054 x 10-27 kg), then the resolution of the mass spectrometer need be of the order of only 1 part in 104. In contrast, to determine the mass of individual atoms so that the mass defect can be determined, the precision must approach 1 part in 1010. With a mass spectrometer of this precision, molecules of nominally the same mass such as 12C160 (of mass 27.9949mu) can be distinguished from 14N2 (of mass 28.0061mu) and the elemental and isotopic composition of ions of nominal mass less than 1000mu may be determined unambiguously.
ionization and detection methods
The major practical challenge with mass spectrometry is the conversion of a sample into gaseous ions. Typically, less than a milligram of compound is used. Many differen experimental arrangements have been devised to produce gas-phase ions but all suffer from a tendency to fragment the compound of interest. Electron impact ionization (EI) relies on bombarding a sample with high-energy electrons to cause both vaporization and ionization. A disadvantage is that EI tends to induce considerable decomposition in larger molecules. Fast atom bombardment (FAB) is similar to EI, but bombardment o the sample with fast neutral atoms is used to vaporize and ionize the sample; it induces less fragmentation than EL Matrix-assisted laser desorption/ionization (MALDI) is similar to EI, but a short laser pulse is used to the same effect; this technique is particularly effective with polymeric samples. In electrospray ionization (ESI), charged droplets of solution are sprayed into a vacuum chamber where solvent evaporation results in generation of individually charged ions; ESI mass spectrometry is becoming more widely used and is often the method of choice for ionic compounds in solution.
Figure 2. A time of flight (TOF) mass spectrometer. The molecular fragments are accelerated to different speeds by the potential difference and arrive at different times at the detector.
The traditional method of ion separation relies on the acceleration of ions with an electric field and then using a magnetic field to deflect the moving ions: ions with a lower mass-to-charge ratio are deflected more than heavier ions. As the magnetic field is changed, ions with different mass-to-charge ratio are directed on to the detector (Fig. 1). In a time-of-flight (TOF) mass spectrometer, the ions from a sample are accelerated by an elec-tric field for a fixed time and then allowed to fly freely (Fig. 2). Because the force on all the ions of the same charge is the same, the lighter ions are accelerated to higher speeds than the heavier ions and strike a detector sooner. In an ion cyclotron resonance (ICR) mass spectrometer (often denoted FTICR, for Fourier transform-ICR) ions are collected in a small cyclotron cell inside a strong magnetic field. The ions circle round in the magnetic field, effectively behaving as an electric current. Because an accelerated current generates electromagnetic radiation, the signal generated by the ions can be detected and used to establish their mass-to-charge ratio.
Mass spectrometry is most widely used in organic chemistry but is also very useful for the analysis of inorganic compounds. However, many inorganic compounds, such as those with ionic structures or covalently bonded networks (for example SiO2), are not volatile and do not fragment into molecular ion units (even with the MALDI technique) so cannot be analysed by this method. Conversely, the weaker bonding in some inorganic coordina-tion compounds means that they fragment much more easily than organic compounds in the mass spectrometer.
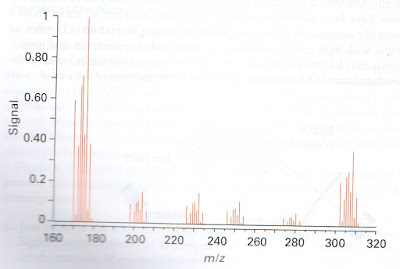
Figure 3. The example of mass spectrum
Interpretation
Figure 3 shows a typical mass spectrum. To interpret a spectrum, it is helpful to detect a peak corresponding to the singly charged, intact molecular ion. Sometimes a peak occurs at half the molecular mass and is then ascribed to a doubly charged ion. Peaks from multi-ply charged ions are usually easy to identify because the separation between the peaks from the different isotopomers is no longer mu but fractions of that mass. For instance, in a doubly charged ion, isotopic peaks are 1/2mu apart, in a triply charged ion they are imu apart, and so on.
In addition to indicating the mass of the molecule or ion that is being studied (and hence its molar mass), a mass spectrum also provides information about fragmentation pathways of molecules. This information can be used to confirm structural assignments. For example, complex ions often lose ligands and peaks are observed that correspond to the complete ion less one or more ligands.
Figure 4. The mass spectrum of sample containing mercury showing the isotropic composition of the atoms
Multiple peaks are observed when an element is present as a number of isotopes (for instance, chlorine is 75.5 per cent 35Cl and 24.5 per cent 37Cl). Thus, for a molecule containg chlorine, the mass spectrum will show two peaks 2mu apart in an intensity ratio of about 3:1. Different patterns of peaks are obtained for elements with a more complex isotropic composition and can be used to identify the presence of an element in compounds of unknown composition. An Hg atom, for instance, has six isotopes in significant abundance (Fig. 4). The actual proportion of isotopes of an element varies according to its geographic source, and this subtle aspect is easily identified with high-resolution mass spectrometers. Thus, the precise determination of the proportions of isotopes can be used to determine the source of a sample.
loading...




0 Response to "Mass spectrometry"
Post a Comment