Almost all the whole of modern chemical industry depends on the development, selection, and application of catalysts. All we can hope to do in this section is to give the brief indication of some of the problems involved. Other than the ones we consider, these include the danger of the catalyst being poisoned by by-products or impurities and economic considerations relating to cost and lifetime.
Many metals are suitable for adsorbing gases, and the genelar order of adsorption strengths decreases along the series O2, C2H2, C2H4, CO, H2, CO2, N2. Some of these molecules adsorb dissociatively (e.g. H2). Transition metal such as Fe, V and Cr show a strong activity towards all these gases, but Mn and Cu are unable to adsorb N2 and CO2, and adsorb H2 only very weakly. Metals towards the left of the Periodic Table (e.g. Mg and Li) can adsorb only the most active gas (O2). These trends are also summarized in Table 1.
Table 1. Properties of Catalysts
Oxidation
Catalytic oxidation is also widely used in industry and in pollution control. Although in some cases it is desirable to achieve complete oxidation (as in the production of nitric acid from ammonia), in others partial oxidation is the aim. For example, the complete oxidation of propene to carbon dioxide and water is wasteful, but its partial oxidation to acrolein, CH2=CHCHO is the start of important industrial processes. Likewise, the controlled oxidations of ethene to ethanol, ethanal (acetaldehyde), and (in the presence of acetic acid or chlorine) to vinyl acetate or vinyl chloride, are the initial stages of very important chemical industries.
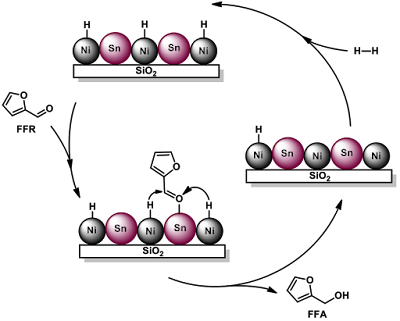
Not all these reactions proceed on a simple metal catalyst, for some depend on oxides of various kinds. The physical chemistry of these surfaces is very obscure, as can be appreciated by considering what happens during the oxidation Of propene, to acrolein, CH2=CHCH3, on bismuth molybdate. The first stage is the adsorbation of the propene molecule with loss of a hydrogen to form the allyl radical, CH2=CHCHO. The oxygen in the surface can transfer to the allyl, leading to the formation of acrolein and its desorption from the surface. The H atom also escapes with a surface O, and goes on to form H2O, which leaves the surface. The surface is left with the charges the oxide ions discarded as they formed O atoms, and these are attacked by O2 molecules in the overlying gas which then chemisorb as oxide ions, so reforming the catalyst. This sequence of events involves great upheavals of the surface, and some materials break up under the stress.
Cracking and reforming
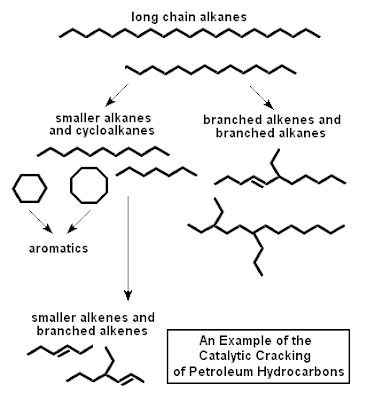
Many of the small organic molecules used in the preparation of all kinds of chemical products come from oil. These small building blocks of polymers, perfumes, and petrochemicals in general, are often cut from the long-chain hydrocarbons squeezed out of the earth. The catalytically induced fragmentation of the long-chain hydrocarbons is called cracking, and is often brought about on silica-alumina catalysts. These act by forming unstable carbonium ions, which fall apart and rearrange to more highly branched isomers. These branched isomers burn more smoothly and efficiently in internal combustion engines, and are used to produce higher 'octane' fuels.
Catalytic cracking has been largely superseded by catalytic reforming using a dual-function catalyst, such as a mixture of platinum and alumina. The platinum provides the metal function, and brings about dehydrogenation and hydrogenation. The alumina provides the acidic function, being able to form carbonium ions.
The sequence of events in catalytic reforming shows up very clearly the complications that must be unravelled if a reaction as important as this is to be understood and improved. The first step is the attachment of the long-chain hydrocarbon by chemisorption to the platinum. In this process first one and then a second H atom is lost, and an alkene is formed. The alkene migrates to an acid site, where it accepts a proton and attaches to the surface as a carbonium ion. This carbonium ion can undergo several different reactions. It can break into two, isomerize into a more highly branched form, or undergo varieties of ring-closure. Then it loses a proton, escapes from the surface, and migrates (possibly through the gas) as an alkene to a metal part of the catalyst where it is hydrogenated. We end up with a rich selection of smaller molecules which can be withdrawn, fractionated, and then used as raw materials for other products.
Many metals are suitable for adsorbing gases, and the genelar order of adsorption strengths decreases along the series O2, C2H2, C2H4, CO, H2, CO2, N2. Some of these molecules adsorb dissociatively (e.g. H2). Transition metal such as Fe, V and Cr show a strong activity towards all these gases, but Mn and Cu are unable to adsorb N2 and CO2, and adsorb H2 only very weakly. Metals towards the left of the Periodic Table (e.g. Mg and Li) can adsorb only the most active gas (O2). These trends are also summarized in Table 1.
Table 1. Properties of Catalysts
Catalyst
|
Function
|
Example
|
Metals
|
Hydrogenation
Dehydrogenation
|
Fe,
Ni, Pt, Ag
|
Semiconducting
oxide and sulphides
|
Oxidation
Desulphurization
|
NiO, ZnO, MgO,
Bi2O3/MoO3
|
Insulating
oxides
|
Dehydration
|
Al2O3,
SiO2, MgO
|
Acids
|
Polymerization
Isomerization
Cracking
Alkylation
|
H3PO4,
H2SO4, SiO2/Al2O3
|
Oxidation
Catalytic oxidation is also widely used in industry and in pollution control. Although in some cases it is desirable to achieve complete oxidation (as in the production of nitric acid from ammonia), in others partial oxidation is the aim. For example, the complete oxidation of propene to carbon dioxide and water is wasteful, but its partial oxidation to acrolein, CH2=CHCHO is the start of important industrial processes. Likewise, the controlled oxidations of ethene to ethanol, ethanal (acetaldehyde), and (in the presence of acetic acid or chlorine) to vinyl acetate or vinyl chloride, are the initial stages of very important chemical industries.
Not all these reactions proceed on a simple metal catalyst, for some depend on oxides of various kinds. The physical chemistry of these surfaces is very obscure, as can be appreciated by considering what happens during the oxidation Of propene, to acrolein, CH2=CHCH3, on bismuth molybdate. The first stage is the adsorbation of the propene molecule with loss of a hydrogen to form the allyl radical, CH2=CHCHO. The oxygen in the surface can transfer to the allyl, leading to the formation of acrolein and its desorption from the surface. The H atom also escapes with a surface O, and goes on to form H2O, which leaves the surface. The surface is left with the charges the oxide ions discarded as they formed O atoms, and these are attacked by O2 molecules in the overlying gas which then chemisorb as oxide ions, so reforming the catalyst. This sequence of events involves great upheavals of the surface, and some materials break up under the stress.
Cracking and reforming
Many of the small organic molecules used in the preparation of all kinds of chemical products come from oil. These small building blocks of polymers, perfumes, and petrochemicals in general, are often cut from the long-chain hydrocarbons squeezed out of the earth. The catalytically induced fragmentation of the long-chain hydrocarbons is called cracking, and is often brought about on silica-alumina catalysts. These act by forming unstable carbonium ions, which fall apart and rearrange to more highly branched isomers. These branched isomers burn more smoothly and efficiently in internal combustion engines, and are used to produce higher 'octane' fuels.
Catalytic cracking has been largely superseded by catalytic reforming using a dual-function catalyst, such as a mixture of platinum and alumina. The platinum provides the metal function, and brings about dehydrogenation and hydrogenation. The alumina provides the acidic function, being able to form carbonium ions.
The sequence of events in catalytic reforming shows up very clearly the complications that must be unravelled if a reaction as important as this is to be understood and improved. The first step is the attachment of the long-chain hydrocarbon by chemisorption to the platinum. In this process first one and then a second H atom is lost, and an alkene is formed. The alkene migrates to an acid site, where it accepts a proton and attaches to the surface as a carbonium ion. This carbonium ion can undergo several different reactions. It can break into two, isomerize into a more highly branched form, or undergo varieties of ring-closure. Then it loses a proton, escapes from the surface, and migrates (possibly through the gas) as an alkene to a metal part of the catalyst where it is hydrogenated. We end up with a rich selection of smaller molecules which can be withdrawn, fractionated, and then used as raw materials for other products.
loading...
0 Response to "The examples of catalysis"
Post a Comment