Some of the terminology used to describe the rate of a catalytic reaction and its mechanism are introduced as follows. This is urgent to understand the important words used in catalytic process before we discuss the mechanism of catalytic reaction.
Energetic
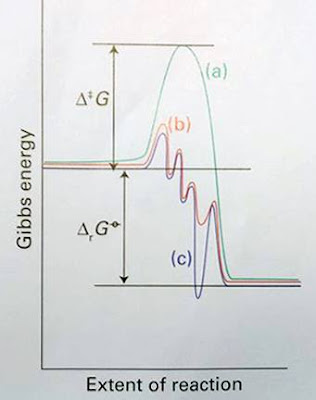
A catalyst increases the rates of processes by introducing new pathways with lower Gibbs energies of activation, ∆G. We need to focus on the Gibbs energy profile of a catalytic reaction, not just the enthalpy or energy profile, because the new elementary steps that occur in the catalyzed process are likely to have quite different entropies of activation. A catalyst does not affect the Gibbs energy of the overall reaction, ∆rG, because G is a state function. The difference is illustrated in figure 1, where the overall reaction Gibbs energy is the same in both energy profiles. Reactions that are thermodynamically unfavourable cannot ge made favourable by a catalyst.
Figure 1. Schematic representation of energetics of a catalytic cycle. The uncatalysed reaction (a) has a higher ∆G than a step in catalysed reaction (b). The Gibbs energy of the overall reaction, ∆rG , is the same for routes (a) and (b). The curve (c) shows the profile for a reaction mechanism with an intermediate that is more stable than the product
Figure 1 also shows that the Gibbs energy profile of a catalyzed reaction contains no high peaks and no deep troughs. The new pathway introduced by the catalyst change the mechanism of the reaction to one with a very different shape and with lower maxima. However, an equally important point is that stable or nonlabile catalytic intermediates do not occur in the cycle. Similarly, the product must be released in a thermodynamically favourable step. If, as shown by the blue line in figure1, a stable complex were formed with the catalyst, it would turn out to be the product of the reaction and the cycle would terminate. Similarly, impurities may suppress catalyst by coordinating strongly to catalytically active sites and act as catalyst poisons.
Catalytic cycle
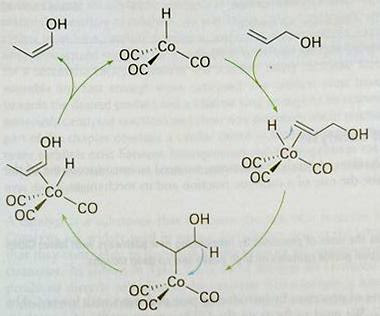
The essence of catalysis is a cycle of reactions in which the reactants are consumed, the products are formed, and the catalytic species is regenerated. A simple example of catalytic cycle involving a homogeneous catalyst is the isomerization of prop-2-en-1-ol (allyl alcohol (CH2=CHCH2OH)) to prop-1-en-1-ol (CH3CH=CHOH) with the catalyst [Co(CO)3H]. The first step is the coordination of the reactant to the catalyst. That complex isomerized in the coordination sphere of the catalyst and goes on to release the product and reform the catalyst (figure 2). Once release the prop-1-en-1-ol tautomerises to propanal (CH3CH2CHO). As with all mechanisms, this cycle has been proposed on the basis of a range of information like that summarized in figure 3. However, the elucidation of catalytic machanisms is complicated by the occurence of several delicately balanced reactions, which often cannot be studied in isolation.
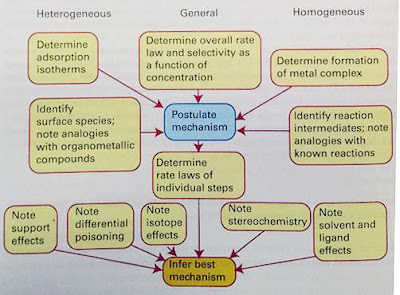
Two stringent test of any purposed mechanism are the determination of rate laws and the elucidation of streochemistry. If intermediates are postulated, their detection by magnetic resonance and IR spectroscopy also provides support. If specific atom-transfer steps are proposed, then isotopic tracer studies may serve as a test. The influences of different ligands and different substrates are also sometimes informative. Although rate date and the corresponding laws have been determined for many overall catalytic cycles, it is also necessary to determine rate laws for the individual steps in order to have reasonable confidence in the mechanism. However, because of experimental compilations, it is rare that catalytic cycle are studied in this detail.
Figure 2. The catalytic cycle for the isomerization of prop-2-en-1-ol to prop-1-en-1-ol
Figure 3. The determination of catalytic mechanism
Catalytic efficiency and lifetime
The turnover frequency, f, is often used to express the effeciency of catalyst. For the conversion of A to B catalysed by Q and with a rate v. Then provide the rate of uncatalysed reaction is negligible, the turnover frequency is :
A highly active catalyst, one that results in a fast reaction even in low concentrations, has a high turnover frequency. In heterogeneous catalysis, the reaction rate is expressedin terms of the rate of change in the amount of product (in place of concentration) and the concentration of catalyst is replaced by the amount present. The determination of the number of active sites in a heterogeneous catalyst is particularly challenging, and often denominator [Q] in equation above is replace by the surface area of the catalyst.
The turnover number is the number of cycles for which a catalyst survives. If it is to be economically viable, a catalyst must have a large turnover number. However, it may be destroyed by side reactions to the main catalytic cycle or by the presence of small amounts of impurities in the starting materials (the feedstock). For example, many alkene polymerization catalysts are destryed by O2, so in the synthesis of polyethene (polyethylene) and polypropene (polypropylene) the concentration of O2 in the ethene or propene feedstock should be no more than a few parts per billion.
Some catalysts can be regenerated quite readily. For example, the supported metal catalysts used in the reforming reactions that convert hydrocarbons to high-octane gasoline become cevered with carbon because the catalytic reaction is accompanied by a small amount of dehydrogenaytion. These supported metal particles can be cleaned by interrupting the catalytic process periodically and burning off the accumulated carbon.
Selectivity
A selective catalyst yields a high proportion of the desired product with minimum amounts of side products showed in analytical result. In industry, the is considerable economic incentive to develop selective catalysts. For example, when metallic silver is used to catalyse the oxidation of ethene with oxygen to produce oxirane (ethylene oxide), the reaction is accompanied by the more thermodinamically favoured but undesrable formation of CO2 and H2O. This lack of selective increases the consumtion of ethene, so chemists are constantly trying to device a more selective catalyst for oxirane synthesis. Selectivity can be ignored in only a very few simple inorganic reactions, where there is esentially only one thermodynamically favourable product, as in the formation NH3 from H2 dan N2. One area where selectivity is of considerable and growing importance is asymmetric synthesis, where only one enantiomer of particular compound is required and catalysts may be designed to produce one chiral form in preference to any others.
Question:
Which of the following constitute genuine example of catalysis and which do not? Present your reasoning.
(a). The addition of H2 to C2H4 when the mixture is brought into contact with finely devided platinum.
(b). The reaction of an H2 / O2 gas mixture when an electrical arc is struck
(c). The combination of N2 gas with lithium metal to produce Li3N, which then reacts with H2O to produce NH3 and LiOH.
Energetic
A catalyst increases the rates of processes by introducing new pathways with lower Gibbs energies of activation, ∆G. We need to focus on the Gibbs energy profile of a catalytic reaction, not just the enthalpy or energy profile, because the new elementary steps that occur in the catalyzed process are likely to have quite different entropies of activation. A catalyst does not affect the Gibbs energy of the overall reaction, ∆rG, because G is a state function. The difference is illustrated in figure 1, where the overall reaction Gibbs energy is the same in both energy profiles. Reactions that are thermodynamically unfavourable cannot ge made favourable by a catalyst.
Figure 1. Schematic representation of energetics of a catalytic cycle. The uncatalysed reaction (a) has a higher ∆G than a step in catalysed reaction (b). The Gibbs energy of the overall reaction, ∆rG , is the same for routes (a) and (b). The curve (c) shows the profile for a reaction mechanism with an intermediate that is more stable than the product
Figure 1 also shows that the Gibbs energy profile of a catalyzed reaction contains no high peaks and no deep troughs. The new pathway introduced by the catalyst change the mechanism of the reaction to one with a very different shape and with lower maxima. However, an equally important point is that stable or nonlabile catalytic intermediates do not occur in the cycle. Similarly, the product must be released in a thermodynamically favourable step. If, as shown by the blue line in figure1, a stable complex were formed with the catalyst, it would turn out to be the product of the reaction and the cycle would terminate. Similarly, impurities may suppress catalyst by coordinating strongly to catalytically active sites and act as catalyst poisons.
Catalytic cycle
The essence of catalysis is a cycle of reactions in which the reactants are consumed, the products are formed, and the catalytic species is regenerated. A simple example of catalytic cycle involving a homogeneous catalyst is the isomerization of prop-2-en-1-ol (allyl alcohol (CH2=CHCH2OH)) to prop-1-en-1-ol (CH3CH=CHOH) with the catalyst [Co(CO)3H]. The first step is the coordination of the reactant to the catalyst. That complex isomerized in the coordination sphere of the catalyst and goes on to release the product and reform the catalyst (figure 2). Once release the prop-1-en-1-ol tautomerises to propanal (CH3CH2CHO). As with all mechanisms, this cycle has been proposed on the basis of a range of information like that summarized in figure 3. However, the elucidation of catalytic machanisms is complicated by the occurence of several delicately balanced reactions, which often cannot be studied in isolation.
Two stringent test of any purposed mechanism are the determination of rate laws and the elucidation of streochemistry. If intermediates are postulated, their detection by magnetic resonance and IR spectroscopy also provides support. If specific atom-transfer steps are proposed, then isotopic tracer studies may serve as a test. The influences of different ligands and different substrates are also sometimes informative. Although rate date and the corresponding laws have been determined for many overall catalytic cycles, it is also necessary to determine rate laws for the individual steps in order to have reasonable confidence in the mechanism. However, because of experimental compilations, it is rare that catalytic cycle are studied in this detail.
Figure 2. The catalytic cycle for the isomerization of prop-2-en-1-ol to prop-1-en-1-ol
Figure 3. The determination of catalytic mechanism
Catalytic efficiency and lifetime
The turnover frequency, f, is often used to express the effeciency of catalyst. For the conversion of A to B catalysed by Q and with a rate v. Then provide the rate of uncatalysed reaction is negligible, the turnover frequency is :
A highly active catalyst, one that results in a fast reaction even in low concentrations, has a high turnover frequency. In heterogeneous catalysis, the reaction rate is expressedin terms of the rate of change in the amount of product (in place of concentration) and the concentration of catalyst is replaced by the amount present. The determination of the number of active sites in a heterogeneous catalyst is particularly challenging, and often denominator [Q] in equation above is replace by the surface area of the catalyst.
The turnover number is the number of cycles for which a catalyst survives. If it is to be economically viable, a catalyst must have a large turnover number. However, it may be destroyed by side reactions to the main catalytic cycle or by the presence of small amounts of impurities in the starting materials (the feedstock). For example, many alkene polymerization catalysts are destryed by O2, so in the synthesis of polyethene (polyethylene) and polypropene (polypropylene) the concentration of O2 in the ethene or propene feedstock should be no more than a few parts per billion.
Some catalysts can be regenerated quite readily. For example, the supported metal catalysts used in the reforming reactions that convert hydrocarbons to high-octane gasoline become cevered with carbon because the catalytic reaction is accompanied by a small amount of dehydrogenaytion. These supported metal particles can be cleaned by interrupting the catalytic process periodically and burning off the accumulated carbon.
Selectivity
A selective catalyst yields a high proportion of the desired product with minimum amounts of side products showed in analytical result. In industry, the is considerable economic incentive to develop selective catalysts. For example, when metallic silver is used to catalyse the oxidation of ethene with oxygen to produce oxirane (ethylene oxide), the reaction is accompanied by the more thermodinamically favoured but undesrable formation of CO2 and H2O. This lack of selective increases the consumtion of ethene, so chemists are constantly trying to device a more selective catalyst for oxirane synthesis. Selectivity can be ignored in only a very few simple inorganic reactions, where there is esentially only one thermodynamically favourable product, as in the formation NH3 from H2 dan N2. One area where selectivity is of considerable and growing importance is asymmetric synthesis, where only one enantiomer of particular compound is required and catalysts may be designed to produce one chiral form in preference to any others.
Question:
Which of the following constitute genuine example of catalysis and which do not? Present your reasoning.
(a). The addition of H2 to C2H4 when the mixture is brought into contact with finely devided platinum.
(b). The reaction of an H2 / O2 gas mixture when an electrical arc is struck
(c). The combination of N2 gas with lithium metal to produce Li3N, which then reacts with H2O to produce NH3 and LiOH.
loading...
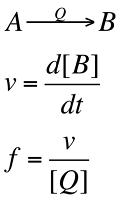
0 Response to "The Important Words of Catalyst"
Post a Comment