Chromatography is a process of analytical chemistry used to separate materials from one another, due to a difference in phase distribution. Materials can exist in three phases: solid, liquid and gas. During chromatography, one component of the mixture is captured while the other components flow fast. Chromatography comes from chroma, the Greek word for color, because extraction of the different materials from the mixtures often results in the appearance of different spots or bands of color. The major types of chromatography are: column, thin layer (TLC) and high pressure liquid (HPLC), all of which are characterized by liquid-solid phase interactions. In addition, gas chromatography (GC or GLPC for gas-liquid partition) involves a mobile gas phase and stationary liquid phase. Paper chromatography involves a liquid-liquid multiple extraction process.
Methods
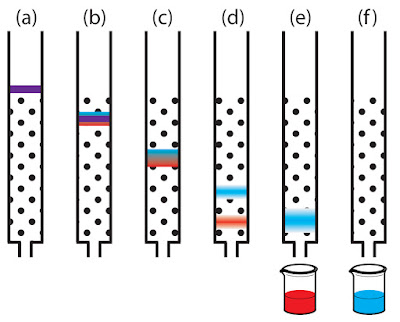
Column chromatography, as the name implies, involves the use of a tall glass column which is packed with finely divided solids such as alumina or silica gel. The mixture is applied at the top and liquid solvent is used to “wash” the components down the length of the column. Different materials travel at different rates because they cling with different intensities to the packing materials. With colored compounds, a series of varicolored bands will form. TLC and paper chromatography are horizontal version of column chromatography. A solid adsorbent is spread in a thin layer on a ridged glass or plastic plate, or on a paper strip. A drop of the mixture is placed at one end along with the solvent and the components of the mixture separate and settle into different spots.
HPLC closely resembles column chromatography. The major differences are that the packing materials are much more densely packed within the column, and a pump is used so the extraction process is done under the high pressure. This is important where the liquids involved have slow diffusion rates. The increased pressure enables the liquid component to be evaporated out in separate units.
Gas chromatography is used to separate volatile compounds whose boiling points differ from each other by less than 0.5oC. It has for all intents and purpose largely replaced functional distillation (another popular extraction method) for separation and purification on a small scale. The mixture to be separated is vaporized and carried along the column by a carrier gas, which is an inert gas such as nitrogen or helium. The lower volatile liquid will be trapped along the packing material, so a partitioning occurs between the gas and liquid phases.
Methods
Column chromatography, as the name implies, involves the use of a tall glass column which is packed with finely divided solids such as alumina or silica gel. The mixture is applied at the top and liquid solvent is used to “wash” the components down the length of the column. Different materials travel at different rates because they cling with different intensities to the packing materials. With colored compounds, a series of varicolored bands will form. TLC and paper chromatography are horizontal version of column chromatography. A solid adsorbent is spread in a thin layer on a ridged glass or plastic plate, or on a paper strip. A drop of the mixture is placed at one end along with the solvent and the components of the mixture separate and settle into different spots.
HPLC closely resembles column chromatography. The major differences are that the packing materials are much more densely packed within the column, and a pump is used so the extraction process is done under the high pressure. This is important where the liquids involved have slow diffusion rates. The increased pressure enables the liquid component to be evaporated out in separate units.
Gas chromatography is used to separate volatile compounds whose boiling points differ from each other by less than 0.5oC. It has for all intents and purpose largely replaced functional distillation (another popular extraction method) for separation and purification on a small scale. The mixture to be separated is vaporized and carried along the column by a carrier gas, which is an inert gas such as nitrogen or helium. The lower volatile liquid will be trapped along the packing material, so a partitioning occurs between the gas and liquid phases.
loading...

0 Response to "The Understanding of Chromatography"
Post a Comment